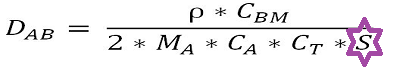Shakshat Virtual Lab 
INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI
CLICK HERE TO SEE THE VIRTUAL WORKSHOP
INTRODUCTION :
Diffusion describes the spread of particles through random motion from regions of higher concentration to regions of lower concentration. The time dependence of the statistical distribution in space is given by the diffusion equation . The concept of diffusion is tied to that of mass transfer driven by a concentration gradient, but diffusion can still occur when there is no concentration gradient (but there will be no net flux). If two gases are inter diffusing with continual supply of fresh gas and removal of the products of diffusion, this diffusion reaches an equilibrium state with constant concentration gradients. This is known as steady state diffusion. If also ther is no total flow in either direction the rates of diffusion of A and B , NA and NB are equal but have opposite sign.
In terms of concentration terms the expression for D is:
..........................(1)
...............................(2)
where:
CA = Molar concentration of A
CB = Molar concentration of B
CT = Total molar concentration
x = Final height from top end of the tube after time t
x0 = Initial height from top end of the tube
x- x0 = Drop in liquid (carbon tetrachloride) level in time t
Usually x0 will not be measured accurately nor is the effective distance for diffusion x ,at time t . Accurate value of (x- x0) are available, however and hence:
Rewriting equation 1 as :
A graph between t/(x- x0) against (x- x0) should yield a straight line with slope
Diffusion Co-efficient: