Shakshat Virtual Lab 
INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI
CLICK HERE TO SEE THE VIRTUAL WORKSHOP
INTRODUCTION :
Solid-liquid mass transfer plays an important role in some industrial operations.The dissolution may occur with or without chemical reaction.In case dissolution is accompanied by solid-liquid reaction ,it is desirable to know the enhancement in the rate of mass transfer due to simultaneous reaction and compare it with the enhancement predicted on the basis of the film and the boundary layer models.Here the system under study is dissolution of benzoic acid in aqueous NaOH solution.The dissolution of a solid in a solution accompanied with instantaneous chemical reaction can be expressed as :
A + nBB => Product
Where A is the solid and B is the liquid phase reactant. Assuming the reaction to be instantaneous so that Aand B don’t co-exist. The mechanism of solid dissolution involves :dissolution of A in liquid followed by its reaction with species B diffusing from the bulk liquid phase at a reaction plane.If the film model is applied to this situation, the enhancement factor, φ defined as the ratio of the solid liquid mass transfer coefficient with reaction kr to the mass transfer coefficient without reaction k given by

And for boundary layer model it is :
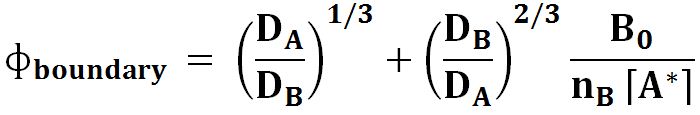
OBJECTIVE :
To Study the dissolution of benzoic acid in aqueous NaOH solution.
To Compare the observed enhancement factor for mass transfer with those predicted by film and boundary layer model.
Mass transfer with chemical reaction (solid - liquid system) : Dissolution of benzoic acid in aqueous NaOH solution.