Shakshat Virtual Lab 
INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI
Binary Vapour Liquid Equiibrum
PROCEDURE :
Cleaning the Setup : The setup is cleaned to start a new Experiment.
Mixing in given ratios : A mixture of benzene and toluene is prepared and put into the flask.
Power is Supplied : Then the power is supplied and the mixture begins to boil.
Equilibrium is Reached: After some time equilibrium is reached, this is marked by steady temperature in both the phases.
Measuring RI : Samples of condensed vapor and liquid are taken. To calculate the composition we need to measure the refractive index of the sample and look into the chart.
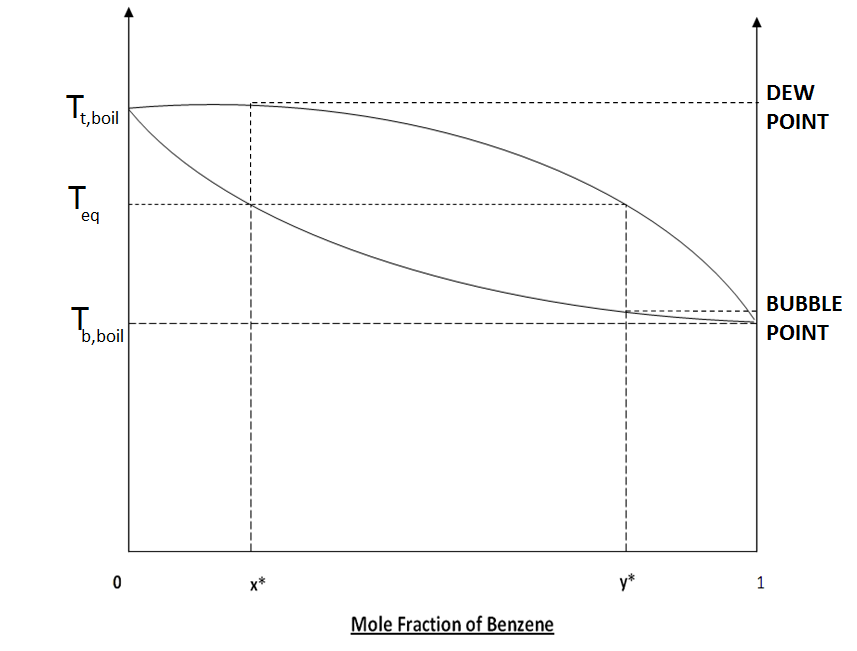
Fig : 1.3 Mole fraction Vs Temperature plot
NOMENCLATURE :
| x* | equilibrium composition of benzene in liquid phase |
| y* |
equilibrium composition of benzene in vapor phase
|
| Teq |
equilibrium temperature
|
| Tt, boil |
Boiling temperature of pure Toluene
|
| Tb, boil |
Boiling temperature of pure Benzene
|
| a |
Relative Volatility
|